so42- lewis structure|SO42 Lewis Structure, Molecular Geometry, : Pilipinas SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It .
We would like to show you a description here but the site won’t allow us.
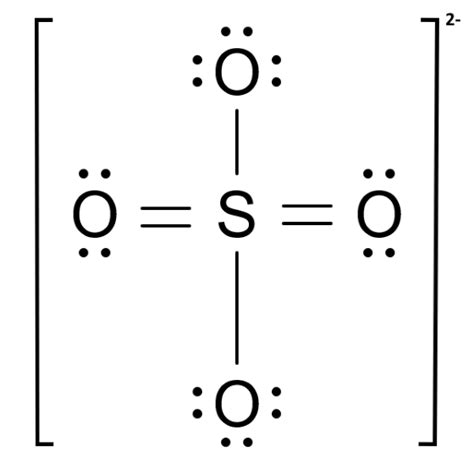
so42- lewis structure,A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb.

Learn how to draw the lewis structure of SO42- ion step by step using total valence electrons concept. See the stable structure with charges minimized and resonanc.
A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geo. Learn how to draw the Lewis structure of sulfate ion (SO42-) with formal charge calculation and VSEPR theory. Find out the molecular geometry, hybridization, and polarity of SO42- with examples and .
Sulfur make six bonds in this Lewis Structure. Two of the oxygens are single-bonded and two are double-bonded. The reason is FORMAL CHARGE and the fact that sulfur doesn't have to follow the.
so42- lewis structure SO42 Lewis Structure, Molecular Geometry, SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It .
so42- lewis structureLewis Structure for SO4 2- (Sulfate Ion) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and .
Learn how to draw the Lewis structure of SO4 2- (sulfate ion), a chemical species with intriguing properties. Follow the steps to determine valence electrons, choose the central . Learn how to draw the Lewis structure of sulfate ion (SO42-), a common anion in chemistry. Follow the steps to calculate valence electrons, indicate lone pairs, assign formal charges, and minimize .
Learn how to draw the dot structure for the sulfate ion, SO42-, with 32 valence electrons. Follow the steps to check the formal charges, use double bonds, and add brackets for . 5 Steps to Draw the Lewis Structure of SO4 2- ion Step #1: Calculate the total number of valence electrons. Here, the given ion is SO4 2-.In order to draw the lewis structure of SO4 2-ion, first of all you have to find the total number of valence electrons present in the SO4 2-ion. (Valence electrons are the number of electrons present in the . La structure SO4 2- Lewis a un atome de soufre (S) au centre qui est entouré de quatre atomes d’oxygène (O). Il y a 2 liaisons simples et 2 doubles liaisons entre l’atome de Soufre (S) et chaque atome d’Oxygène (O). Il y a 2 paires libres sur les atomes d’oxygène à double liaison (O) et 3 paires libres sur les atomes d’oxygène .
Steps of drawing SO4 2- lewis structure Step 1: Find the total valence electrons in SO4 2- ion. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any . Hi Everyone! For today’s video, we are going to do SO42- Lewis Structure. It is a chemical formula for Sulfate ions. To determine its Lewis Structure, we fir.
The initial step in drawing the SO 4 2-Lewis structure is to calculate the total number of valence electrons present in the molecule. Since sulfur and oxygen belong to group 16 of the periodic table, they each have six valence electrons. As SO 4 2-contains one sulfur atom and four oxygen atoms, the total number of valence electrons can be .SO42 Lewis Structure, Molecular Geometry, There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance . The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of electrons around it. The formal charge of an atom is equal to the number of valence electrons, N v.e. minus the number of unshared electrons, N us.e. and half of the bonding electrons, ½ N .
To determine the number of lone pairs and bonding pairs of electrons for SO4 2- we first need to draw as valid Lewis Structure. Once we have a Lewis Structur.
Understanding the Lewis Structure of SO4 2-The first subtitle focuses on explaining the fundamental concepts behind the Lewis structure of the sulfate ion (SO4 2-) in the context of Mathematics education. It aims to provide a clear understanding of how to represent the molecule using Lewis dot diagrams.
For the arrangement HCN, the Lewis structure: H–C\(\equiv\)N: The formal charges work out as follows: For the arrangement HNC, the Lewis structure: H–N\(\equiv\)C: The formal charges work out as follows: Both Lewis structures have a net formal charge of zero, but note that the formal charges on the first structure are all zero! .
Dibujo de la Estructura de Lewis del [SO4]-2 (Ion Sulfato) Saltar al contenido . Inicio; Gases. Ley de Dalton de las Presiones Parciales; Teoria Cinetica Molecular De Los Gases; Ley de Boyle – Graficas – Formulas .

The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species. These bonds can .
Lewis Dot of the Sulfate Ion. SO 4 2-Back: 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for . The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [SO 4] 2- is to count the total valence electrons present in the concerned elemental atoms.. There are two different elements present in the sulfate .Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. The Lewis structure for sulfate is dependent on the formal charge of sulfur. Sulfates molecular formula is SO42-. Two double bonds and two single bonds are used to link oxygen to the sulfur atom.What is the Lewis structure for SO4 2-? The Lewis Dot Structure for SO 4 2-: The sulfate anion (SO 4 2-) results from the complete ionization of sulfuric acid (H 2 SO 4). The representation of the valence electron distribution is properly shown by .
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO42- ion. In lewis structure, there should be charges on atoms.
so42- lewis structure|SO42 Lewis Structure, Molecular Geometry,
PH0 · SO42 Lewis Structure, Molecular Geometry,
PH1 · SO42
PH2 · SO4 2
PH3 · Lewis Structure of SO4(2
PH4 · Lewis Structure for SO4 2
PH5 · How to Draw the Lewis Structure for the Sulfate Ion
PH6 · How to Draw the Lewis Dot Structure for SO4 2